Abstract
The efficacy of SARS-CoV2 vaccines in preventing COVID-19 is less beneficial in cancer populations. We have previously shown that myeloproliferative neoplasms (MPNs), including chronic myeloid leukemia (CML), essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF), demonstrate impaired T-cell responses compared to healthy populations 1 month after BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna) SARS-CoV2 vaccination. We now assess whether serologic and T-cell responses 1 month after vaccination are associated with subsequent SARS-CoV2 infection, and evaluate T-cell responses at later timepoints, including after a booster vaccination.
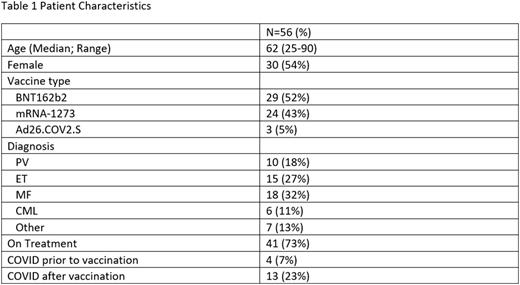
Patients with a known MPN diagnosis presenting at Massachusetts General Hospital and eligible for SARS-CoV2 vaccination were recruited. All participants gave informed consent, and the study protocol was approved by the Institutional Review Board. 56 MPN patients were enrolled and vaccinated. Table 1 shows patient characteristics. Peripheral blood samples were collected at baseline (N=21), 1 (N=28), 6 (N=29), or 12 months (N=5) after initial vaccine series (2 doses of BNT162b2 and mRNA-1273, 1 dose of Ad26.COV2.S), and 1 month after booster (N=25). Of 56 patients, 4 had prior SARS-CoV2 infection before vaccination, and 13 (23%) subsequently developed SARS-CoV2 infection after vaccination, of which 4 infections occurred after booster. The median time from first vaccine to infection was 289 days. COVID-19 was mild in 15 patients (outpatient treatment only) and moderate in 2 (requiring hospitalization); no patient had severe disease or critical illness. Five patients received treatment for COVID-19.
We have previously described serologic and T-cell responses in MPN patients 1 month after initial vaccination. Here we evaluated the association between 1-month immunologic responses and subsequent COVID-19 in 25 patients with available samples. Patients with known COVID-19 prior to vaccination were excluded from analysis. 7/25 patients developed COVID-19. Quantitative anti-spike IgG concentrations 1 month after vaccination were similar in patients who developed or did not develop COVID-19 (534 vs 610 binding antibody units [BAU]/mL; p=0.75). Similarly, we found no differences in anti-spike spot-forming units (SFUs) on IFNγ ELISPOT assays in patients with or without subsequent COVID-19 (median 85 vs 37; p=0.11). Patients who had negative IFNγ release on whole-blood assay also did not have a higher rate of subsequent COVID-19 compared to those with positive responses (61% vs 29%; p=0.20).
We now also describe T-cell responses in MPN patients at later timepoints. In patients with paired samples available (N=13), median anti-spike SFUs on IFNγ ELISPOT assays 6 months after vaccination remained significantly higher compared to pre-vaccination baseline levels (median difference 25.5; p<0.01). Median SFUs declined from 1 to 6 months after vaccination, although this was not significant (N=16, median difference -4.75, p=0.27). The median SFUs in MPN patients with post-booster samples was 30, and 20/24 (83%) of these patients were considered positive ELISPOT responders, as defined as >6 SFUs per 2.5x105 peripheral blood mononuclear cells to spike protein peptide pools. MF patients had significantly lower SFUs compared to other MPN subtypes after booster (p=0.042). In addition, older age was significantly associated with lower SFUs after booster (p=0.011). Lower SFUs were seen in MF versus other MPN patients even when controlling for age in a linear model (p=0.025). There were no significant differences in post-booster SFUs when comparing by gender, vaccine type, MPN treatment, or time from booster to sample collection.
In our cohort of 56 MPN patients, we found a 23% rate of SARS-CoV2 infection after vaccination. Most infections were mild, and we found no associations between subsequent infection and immunologic responses 1 month after initial vaccination, although analysis is limited by the small sample size. Longitudinal data suggests that immunologic responses are durable at 6 months, and overall T-cell responses after booster were high at 83%. MF patients may be less protected after booster given lower T-cell responses seen compared to other MPNs. Cell profiling and serologic analysis is underway to generate further insight on long-term immunologic responses to SARS-CoV2 vaccination in MPNs.
Disclosures
Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. Maus:Arcellx: Consultancy; Astellas: Consultancy; AstraZeneca: Consultancy; Atara: Consultancy; Bayer: Consultancy; BMS: Consultancy; Cabaletta Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cellectis: Consultancy, Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Consultancy, Research Funding; In8bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Intellia: Consultancy; GSK: Consultancy; Kite Pharma: Consultancy, Research Funding; Micromedicine/BendBio: Consultancy; Neximmune: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy, Research Funding; Oncternal: Consultancy, Current holder of stock options in a privately-held company; Sanofi: Consultancy; TCR2: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees; Tmunity: Consultancy; WindMIL: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Promab: Patents & Royalties: held by Massachusetts General Hospital; Novartis: Patents & Royalties: held by University of Pennsylvania; 2SeventyBio: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Century Therapeutics: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Allogene: Consultancy; Adaptimmune: Consultancy; Agenus: Consultancy. Hobbs:Abbvie Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Constellation: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; PI, Research Funding; Pfizer: Other: Advisor or review panel participant; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Pharmaxis: Other: Advisor or review panel participant; Keros: Other: Advisor or review panel participant; Bristol Myers Squibb Co./Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Bayer: Research Funding; Incyte: Other: Advisor or review panel participant; PI, Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal